Take a look at the graphs—perhaps we are over the peak for the spread of H1N1 (swine) flu. Especially, because vaccination is beginning for the the most vulnerable.
Eighteen influenza-associated pediatric deaths were reported. Fifteen of these deaths were associated with 2009 influenza A (H1N1) virus infection and three were associated with an influenza A virus for which the subtype was undetermined.
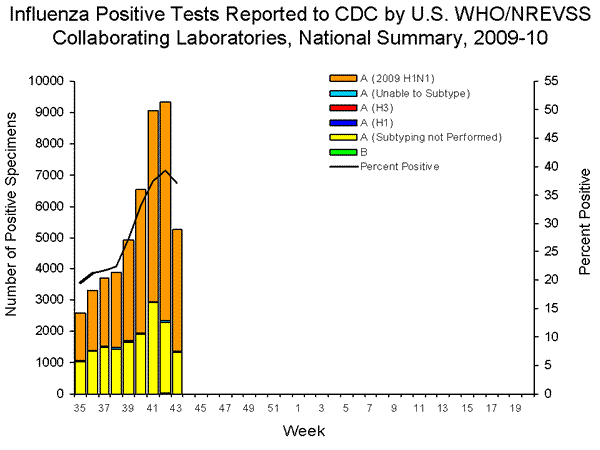
View WHO-NREVSS Regional Bar Charts| View Chart Data | View Full Screen
Pneumonia and Influenza Hospitalization and Death Tracking:
This new system was implemented on August 30, 2009, and replaces the weekly report of laboratory confirmed 2009 H1N1-related hospitalizations and deaths that began in April 2009. Jurisdictions can now report to CDC counts of hospitalizations and deaths resulting from all types or subtypes of influenza, not just those from 2009 H1N1 influenza virus. To allow jurisdictions to implement the new case definition, counts were reset to zero on August 30, 2009. From August 30 – October 31, 2009, 17,838 laboratory-confirmed influenza associated hospitalizations and 672 laboratory-confirmed influenza associated deaths were reported to CDC. CDC will continue to use its traditional surveillance systems to track the progress of the 2009-10 influenza season
Antigenic Characterization:
CDC has antigenically characterized one seasonal A (H1N1), two A (H3N2) and 239 2009 influenza A (H1N1) viruses collected since September 1, 2009.
One seasonal influenza A (H1N1) viruses was tested and is related to the influenza A (H1N1) component of the 2009-10 Northern Hemisphere influenza vaccine (A/Brisbane/59/2007).
Both influenza A (H3N2) viruses tested showed reduced titers with antisera produced against A/Brisbane/10/2007, the 2009-2010 Northern Hemisphere influenza A (H3N2) vaccine component, and were antigenically related to A/Perth/16/2009, the WHO recommended influenza A (H3N2) component of the 2010 Southern Hemisphere vaccine formulation.
Two hundred thirty-eight (99.6%) of 239 2009 influenza A (H1N1) viruses tested are related to the A/California/07/2009 (H1N1) reference virus selected by WHO as the 2009 H1N1 vaccine virus and one virus (0.4%) tested showed reduced titers with antisera produced against A/California/07/2009.
Annual influenza vaccination is expected to provide the best protection against those virus strains that are related to the vaccine strains, but limited to no protection may be expected when the vaccine and circulating virus strains are so different as to be from different lineages. Antigenic characterization of 2009 influenza A(H1N1) viruses indicates that these viruses are only distantly related antigenically and genetically to seasonal influenza A(H1N1) viruses, suggesting that little to no protection would be expected from vaccination with seasonal influenza vaccine. It is too early in the influenza season to determine if seasonal influenza viruses will circulate widely or how well the vaccine and circulating strains will match.
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 43, 7.4% of all deaths reported through the 122-Cities Mortality Reporting System were due to P&I. This percentage was above the epidemic threshold of 6.7% for week 43. Including week 43, P&I mortality has been above threshold for five consecutive weeks.
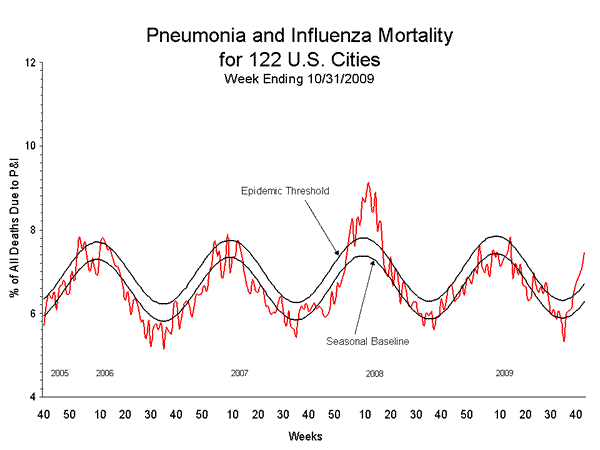
For more details and studies go to: CDC - Seasonal Influenza (Flu) - Weekly Report: Influenza Summary Update

No comments:
Post a Comment